Dear colleagues,
This is my usual selection of highlights on the changes made to the medical device regulations and trends across Russia and the Eurasian Union over the last month:
- Some updates on the Eurasian Medical Device Regulations
On 19 April 2019, the Eurasian Commission published Regulation No. 62 ‘On the classification of the areas of intended use for medical devices’ (link in Russian). This third-level regulation document provides a classification system for the intended use of the medical devices that should be used by the applicant in the preparation of documents for the registration dossier.
Earlier, on 3 April 2019, the Eurasian Economic Commission (EEC) published a draft decision ‘On the rules for assessing and authorizing inspecting organizations to conduct QMS inspections.’ (Link in Russian)
According to the official comment in the published document, the EEC does not support the approach proposed earlier, whereby only government bodies can act as inspecting organisations. The proposed draft document contains the requirements and criteria for the QMS inspection organisations, a description of its accreditation procedure (45 days), as well as the subsequent scheduled and unscheduled government audits (at least once over a two-year period).
It should be recalled that on 16 March 2019, it was the end of the transition period which provided manufacturers with a delay in conducting QMS inspections for Eurasian registration.
- The Russian regulator announced a decrease in the number of registration refusals in 2018
On 26 April 2019, the Russian healthcare regulator, Roszdravnadzor, published a video with its annual report for the year 2018. It cites a 38% decrease in the number of registration rejections during last year when compared to the year 2017: 1342 new medical devices were approved and 398 registration applications were rejected in 2018.
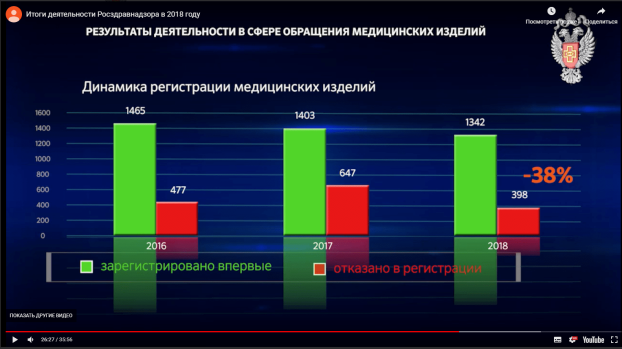
Screenshot from the Roszdravnadzor annual report 2018 shows 38% decrease in number of medical device registration refusals compared to previous year : http://www.roszdravnadzor.ru/news/16631
In addition, the regulator announced its priorities for regulations concerning medical devices in 2019 as being: an introduction of the unified database of patients with implanted medical devices; the development of labelling approaches for medical devices; and the organisation and carrying out of activities to inspect the manufacturers of medical devices according to the Eurasian regulation model.
- Kazakhstan has amended the requirements for inspections
On 10 April 2019, the government of Kazakhstan published the order No. ДР DSM-26 ‘On Approval of the Rules for Inspection in the Sphere of Circulation of Medicinal Products, and Medical Equipment’ (Link in Russian). The document updates the previously established rules and requirements for conducting inspections of manufacturers of pharmaceutical products and medical devices in accordance with Kazakh legislation.
*****
Thank you for following my blog; it is a non-commercial project with the objective of making Russian and Eurasian medical device regulations clearer. You are able to receive updates directly to your email via the ‘Follow’ button on the toolbar.