Dear fellow colleagues and group members,
As the year is coming to a close, in my final post of 2016 I would like to summarise the most remarkable and important updates that have taken place in medical device regulations in Russia and the Eurasian Union this year. Thus, here is the 2016 Russian regulatory environment at a glance:
- First Year of the Eurasian Union Medical Device Harmonised Regulation Model
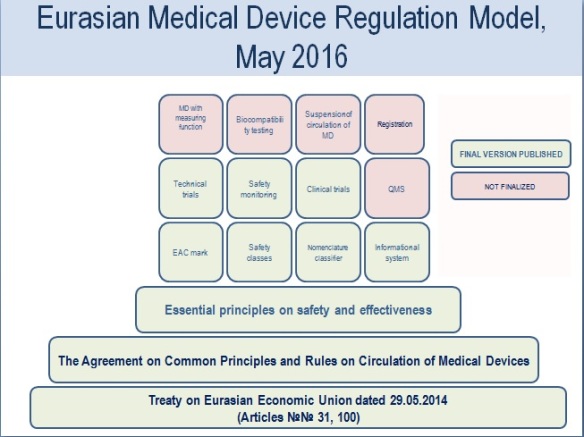
On January 1st 2016 the Agreement On the Common Principles and Rules of Circulation of Medical Devices in Eurasian Economic Union (EEU) officially came into force. Nevertheless, in practice, the common harmonised Eurasian system is still not working.
During the year, the Eurasian Economic Commission (EEC) developed second-level regulations. At the end of the year, twelve out of thirteen legal documents were approved, including new approval (registration) rules for medical devices in the Eurasian Union. One of the most discussed of these second-level guidelines are the requirements for a quality management system for medical devices. Manufacturers are expected to implement mandatory quality audits for most medical device manufacturers for registration within the EEU in 2018.
In addition to the second-level regulations in 2016, the EEC published twelve drafts of third level documents for the above mentioned agreement (e.g. list of voluntary standards, requirements for technical maintenance, recommendations for categorising borderline medical products, recommendations on the content and structure of the registration file). These have currently not been discussed.
In November 2016, the Russian medical device trade association ― IMEDA ― together with the MedTech Europe Associations, held the Round Table “Common Market of Medical Devices: Supranational Regulation Model” in Moscow. Representatives of the EEC, together with European regulators, shared practices and discussed fundamental approaches to forming the common market of the MDs.
According to the Agreement, today all member states of the Eurasian Union (Russia, Belarus, Kazakhstan, Armenia and Kyrgyzstan) are in a transitional period. This Agreement allows developing a Eurasian medical device regulation model, in parallel with local medical device regulations of the member states. This transition period is set to end in 2021.
- Russia Continues Clear Trend of Supporting Local Medical Device Manufacturers
In 2016, the Russian government has continued its course on import substitution, which started as a “crisis management plan” in 2014.In December 2016, the Russian government published Resolution #1268 (link in Russian) and significantly extended the list of certain medical devices that originate from foreign manufacturers. This was initially implemented in February 2015 in the famous Resolution #102, which allowed the rejection of applications for tenders of foreign manufacturers of medical devices on the list in case if two (or more) similar products manufactured in Russia, Belarus or Kazakhstan were proposed. However, in practice, Russian device manufacturers included in the list had to obtain a special ST-1 certificate (not a simple process), in order to prove their products belonged in a “local” category.
Another measure intended to help develop the Russian assembly of complex medical equipment is the amendment of the Russian Tax code. This was implemented in October 2016. It provided support to Russian medical device manufacturers, exempting them from paying value added tax (VAT) for the importation of components and raw material for the manufacturing process in Russia.
Another example is a message from the Russian Federal Antimonopoly Service published in September 2016. They raised a sensible topic for foreign manufacturers of in-vitro diagnostics (IVD) systems and started the discussion to replace “closed” IVD systems with open ones.
- Regulation of Implantable Medical Devices is Continuing to Develop
Price regulations of implantable medical devices ― an initiative that started in 2015 ― had a number of updates in 2016:
In January 2016, the Russian government published Resolution #1517 (link in Russian) titled “On state regulations of prices of medical devices included in the list”, which set the methodology for how mark-ups on such devices should be calculated.
Since February 2016, the Russian medical device regulator Roszdravnadzor has started requesting from manufacturers of implantable medical devices and Russian authorised representatives information about weighted average prices for their products. This uses a newly created electronic database.
In July 2016, the Russian Ministry of Health published guidelines for determining the maximum amount of wholesale mark-up on medical devices implanted in the human body.
In August 2016, the Russian government postponed all deadlines stipulated by the Resolution No. 1517 for one year:
-Deadline for registration of maximum sale prices of implantable devices: delayed until 15 July 2017.
-Deadline for the establishment of the regional authorities of maximum wholesale mark-ups to the actual selling prices of the implantable medical devices: delayed until 1 September 2017.
-Deadline for the proposals by the Russian Ministry of Health in collaboration with other concerned agencies requiring submissions of the government proposals on the agreed upon procedure of re-registration of maximum sale prices: delayed until 1 October 2017.In October 2016, the Russian government extended the list of medical devices that can be implanted into the human body, subject of state healthcare programmes (link in Russian). Compared to the previous version of the list, 160 new medical devices have been added (more than 360 in summary) and some old devices have been removed (devices that were not implantable, as classified according to current nomenclature).
- Development of Local Medical Device Regulations in Russia
The Russian medical device registration process continues to perplex regulatory professionals due to lengthy review times and a high number of rejections. Here are some developments that took place in 2016:
In July 2016 the Russian Ministry of Health published requirements for the technical documentation and instruction for the use (IFU) of medical devices. This clarifies the full list of the information required in the technical file and IFU for medical devices and in-vitro devices.
In August 2016 the Russian Ministry of Health implemented a mandatory procedure for medical device manufacturers to submit amendments in the registration dossier for approved medical devices. This is in cases where technical documentation has been changed.
In October 2016, the Russian Ministry of Health published several draft documents (link in Russian) that are intended to implement significant changes in key medical device regulations. These include simplification of the registration process for IVD products, mandatory safety reporting for high class risk medical devices, and the allowance of official consultancy from Russian medical device regulators. Currently amendments have not been approved; discussions are due to start at the beginning of 2017.
Since August 2016 there has been vivid discussion about a possible extension of the deadline for the procedure of administrative replacements of “old” forms of the registration certificates; nevertheless as of end of the day the 29th December 2016, this suggestion has not been not approved.
- Development of Local Medical Device Regulations in Kazakhstan
Compared to Russia, the medical device approval process in Kazakhstan remains quite predictable. However, several significant changes in the regulations in 2016 should be highlighted:
Since mid-2016 the Order of Kazak Ministry of Health #421(link in Russian) came into force and introduced mandatory regular safety monitoring of medical device manufacturers and their authorised representatives in Kazakhstan. The regulator recommends submitting periodic safety reports that contain information about product incidences and corrective actions both in Kazakhstan and worldwide every three months. This should start from the date of the registration until no later than the tenth day of the month following the report period.
Moreover, in November 2016, the Kazak government published Resolution # 634 (link in Russian) and among others changed the requirements and implemented restrictions for visual advertising of medical devices.
***
That is it for 2016; I would like to thank everyone for following, supporting and contributing to this blog and professional group. I would like to wish you a very happy holiday season and New Year filled with peace, prosperity and great achievements!
Alexey Stepanov
Alexey@medicaldevicesinrussia.com